Bacteriophage therapy for Acinetobacter baumannii
Authors: Hugo Olivera1 and Jeremy J. Barr2
1Centre of Biological Engineering, University of Minho, Braga, Portugal (hugooliveira@deb.uminho.pt)
2School of Biological Sciences, Monash University, Clayton, Australia (jeremy.barr@monash.edu)
Reviewer: Dann Turner
Department of Applied Sciences, University of the West of England, Bristol, UK (dann2.turner@uwe.ac.uk)
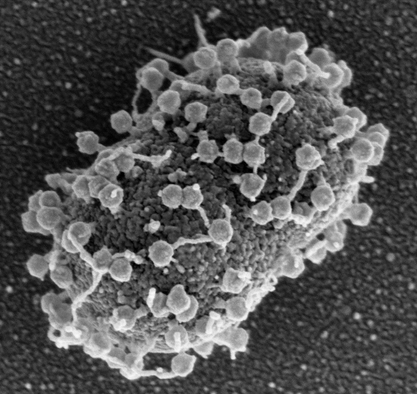
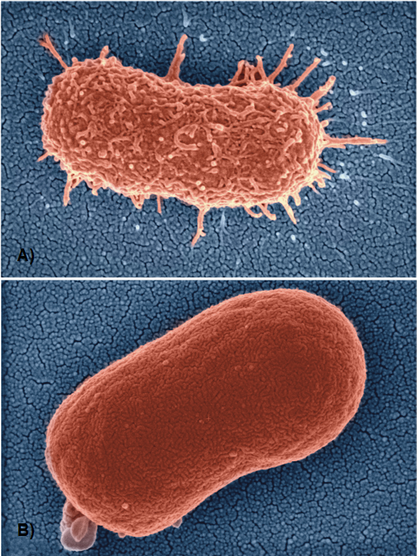
Bacteriophages (phages) are bacterial viruses and the most predominate life form on the planet. These bacterial predators use their tail structures to recognize cognate receptors on bacterial host cells (adsorption step), inject their nucleic acid genomes inside the host (infection step), hijack the bacteria's cellular machinery to replicate (replication step) and finally lyse the host cell to release infectious progeny (lysis step). Due to their mode of action, lytic or virulent phages have been utilized as an alternative therapy against multidrug-resistant bacterial infections. Acinetobacter baumannii has been identified as a priority critical target for the development of new therapeutic options by the WHO, CDC (CDC 2019) (Figure 1). The well-known “Patterson Case” was the first personalized use of phage therapy in the Western World to save the life of a critically ill patient suffering from a systemic, multidrug-resistant A. baumannii infection (Schooley RT et al 2017). Phages infecting Acinetobacter spp. are genetically diverse, being grouped into several subfamilies and genera (Oliveira H et al 2021), and often recognize the Acinetobacter capsule as receptors (Timoshina OY et al 2023, Oliveira H & Costa R et al 2017, Oliveira H et al 2019). As a result of capsule-dependent infection, A. baumannii cells that develop resistance towards phage infection often lose their capsules, becoming less virulent and more susceptible to antimicrobials and the immune system, thereby further extending the clinical efficacy of phage therapy (Altamirano FG et al 2021, Altamirano FG et al 2021) (Figure 2).
Figure 1. Adsorption of anti-Acinetobacter baumannii phage FG02 to the surface of the clinical, multidrug-resistant strain AB900. Figure adapted from Altamirano FG et al 2022. Image taken by Dr Denis Korneev.
Figure 2. A) Scanning electron microscopy image of Acinetobacter baumannii strain A9844 producing capsule layer. B) A. baumannii strain A9844 with a loss-of-function mutation in a core capsule producing gene, which produces a capsule-deficient, phage-resistant, and reduced virulence phenotype. Figure adapted from Altamirano FG et al. 2021. Image taken by Dr Denis Korneev.
To mediate the adsorption and infection steps, some phages possess enzymatic structures, typically displayed on phage tail fibers or base plates, that break down the thick and highly diversified capsular polysaccharide layer surrounding the A. baumannii cell surface (Kenyon JJ & Hall RM 2013, Wyres KL et al 2020, Oliveira H & Costa R et al 2017). These enzymes are called capsular depolymerases and can now be predicted using advanced bioinformatic tools such as PhageDPO (Vieira M et al 2022). Since Acinetobacter capsules represent a major virulence factor, phage-derived depolymerases have potential as anti-virulence agents. Recombinantly expressed depolymerases have been shown to degrade the capsular layer (Figure 3), exposing A. baumannii cells to the host immune system, thereby controlling the infection (Oliveira H et al 2019, Oliveira et al 2019). Although these depolymerases do not kill the cells “per se”, they represent useful anti-virulence agents as evidenced in studies using animal models.
Figure 3. A) Plaque characteristics of Acinetobacter baumannii phages encoding capsular depolymerases. B) Drop test example of an A. baumannii phage and its recombinant depolymerase. Figures adapted from Oliveira H & Costa R et al 2017. Image taken by Hugo Oliveira.
Besides the evident therapeutic effect of phage therapy against drug-resistant infections, there are still some limitations before this technology can broadly applied in the treatment of A. baumannii infections. The forefront limitations are associated with regulatory hurdles for that currently limit the clinical use of phage therapy to compassionate use cases. In addition, A. baumannii phages typically have a very narrow host-range, with many being highly specific for a given capsule type. This is further compounded by the large variation seen across the composition and structure of A. baumannii capsular polysaccharides, with over 200 capsule-types currently listed on the Kaptive database (Cahill SM et al 2022). Therefore, the isolation of novel phages that target diverse receptors other than the capsule, may provide broader host range phages with improved clinical utility. Phages can also be centralized in phage biobanks to allow rapid screening approaches of well-defined phages with demonstrated antimicrobial activity against specific clinical isolates. With the rapid advances in synthetic biology tools, it will be possible to engineer both recombinant phage-derived depolymerases with the ability to recognize new and multiple capsular polysaccharide substrates, and virulent phages with expanded host ranges and enhanced bacterial killing activity through the expression of antimicrobial payloads (e.g., antimicrobial peptides, anti-quorum sensing molecules) (Oliveira H et al 2022, Du J et al 2023, Meile S & Du Jiemin et al 2023). Further research into the discovery, characterization, and application of anti-A. baumannii phages will undoubtedly lead to innovative strategies for the treatment of multidrug-resistant infections which will increase/expand our modern medical “toolbox” against this critically important pathogen.
References
https://www.cdc.gov/drugresistance/biggest-threats.html
Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017 Sep 22;61(10):e00954-17.
doi: 10.1128/AAC.00954-17
Erratum in: Antimicrob Agents Chemother. 2018 Nov 26;62(12)
PMID: 28807909
Oliveira H, Domingues R, Evans B, Sutton JM, Adriaenssens EM, Turner D. Genomic diversity of bacteriophages infecting the genus Acinetobacter. Viruses. 2022 Jan 19;14(2):181.
doi: 10.3390/v14020181
PMID: 35215775
Timoshina OY, Kasimova AA, Shneider MM, Matyuta IO, Nikolaeva AY, Evseev PV, Arbatsky NP, Shashkov AS, Chizhov AO, Shelenkov AA, Mikhaylova YV, Slukin PV, Volozhantsev NV, Boyko KM, Knirel YA, Miroshnikov KA, Popova AV. Friunavirus phage-encoded depolymerases specific to different capsular types of Acinetobacter baumannii. Int J Mol Sci. 2023 May 22;24(10):9100.
doi: 10.3390/ijms24109100
PMID: 37240444
Oliveira H, Costa AR, Konstantinides N, Ferreira A, Akturk E, Sillankorva S, Nemec A, Shneider M, Dötsch A, Azeredo J. Ability of phages to infect Acinetobacter calcoaceticus-Acinetobacter baumannii complex species through acquisition of different pectate lyase depolymerase domains. Environ Microbiol. 2017 Dec;19(12):5060-5077.
Erratum in: Environ Microbiol. 2021 Jun;23(6):3334
PMID: 29076652
Oliveira H, Costa AR, Ferreira A, Konstantinides N, Santos SB, Boon M, Noben JP, Lavigne R, Azeredo J. Functional analysis and antivirulence properties of a new depolymerase from a myovirus that infects Acinetobacter baumannii capsule K45. J Virol. 2019 Feb 5;93(4):e01163-18.
doi: 10.1128/JVI.01163-18
Erratum in: J Virol. 2020 Apr 16;94(9)
PMID: 30463964
Gordillo Altamirano F, Forsyth JH, Patwa R, Kostoulias X, Trim M, Subedi D, Archer SK, Morris FC, Oliveira C, Kielty L, Korneev D, O'Bryan MK, Lithgow TJ, Peleg AY, Barr JJ. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat Microbiol. 2021 Feb;6(2):157-161.
doi: 10.1038/s41564-020-00830-7
PMID: 33432151
Gordillo Altamirano FL, Barr JJ. Unlocking the next generation of phage therapy: the key is in the receptors. Curr Opin Biotechnol. 2021 Apr;68:115-123.
doi: 10.1016/j.copbio.2020.10.002
PMID: 33202354
Kenyon JJ, Hall RM. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLoS One. 2013 Apr 16;8(4):e62160.
doi: 10.1371/journal.pone.0062160
PMID: 23614028
Wyres KL, Cahill SM, Holt KE, Hall RM, Kenyon JJ. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb Genom. 2020 Mar;6(3):e000339.
PMID: 32118530
Vieira M, Duarte J, Domingues R, Oliveira H, Dias O. PhageDPO: Phage depolymerase finder. bioRxiv 2023.02.24.529883.
doi: https://doi.org/10.1101/2023.02.24.529883.
Oliveira H, Mendes A, Fraga AG, Ferreira A, Pimenta AI, Mil-Homens D, Fialho AM, Pedrosa J, Azeredo J. K2 Capsule depolymerase is highly stable, is refractory to resistance, and protects larvae and mice from Acinetobacter baumannii sepsis. Appl Environ Microbiol. 2019 Aug 14;85(17):e00934-19.
doi: 10.1128/AEM.00934-19
Erratum in: Appl Environ Microbiol. 2020 Mar 18;86(7)
PMID: 31227554
Cahill SM, Hall RM, Kenyon JJ. An update to the database for Acinetobacter baumannii capsular polysaccharide locus typing extends the extensive and diverse repertoire of genes found at and outside the K locus. Microb Genom. 2022 Oct;8(10):mgen000878.
PMID: 36214673
Oliveira H, Drulis-Kawa Z, Azeredo J. Exploiting phage-derived carbohydrate depolymerases for combating infectious diseases. Trends Microbiol. 2022 Aug;30(8):707-709.
doi: 10.1016/j.tim.2022.05.002
PMID: 35691880
Du J, Meile S, Baggenstos J, Jäggi T, Piffaretti P, Hunold L, Matter CI, Leitner L, Kessler TM, Loessner MJ, Kilcher S, Dunne M. Enhancing bacteriophage therapeutics through in situ production and release of heterologous antimicrobial effectors. Nat Commun. 2023 Jul 20;14(1):4337.
doi: 10.1038/s41467-023-39612-0
PMID: 37474516
Meile S, Du J, Staubli S, Grossmann S, Koliwer-Brandl H, Piffaretti P, Leitner L, Matter CI, Baggenstos J, Hunold L, Milek S, Guebeli C, Kozomara-Hocke M, Neumeier V, Botteon A, Klumpp J, Marschall J, McCallin S, Zbinden R, Kessler TM, Loessner MJ, Dunne M, Kilcher S. Engineered reporter phages for detection of Escherichia coli, Enterococcus, and Klebsiella in urine. Nat Commun. 2023 Jul 20;14(1):4336.
doi: 10.1038/s41467-023-39863-x
PMID: 37474554
Gordillo Altamirano FL, Kostoulias X, Subedi D, Korneev D, Peleg AY, Barr JJ. Phage-antibiotic combination is a superior treatment against Acinetobacter baumannii in a preclinical study. EBioMedicine. 2022 Jun;80:104045.
doi: 10.1016/j.ebiom.2022.104045
PMID: 35537278