Colony Phase Variation Switching
Authors: Irfan Ahmad and Bernt Eric Uhlin
Department of Molecular Biology, Umeå University, Umeå, Sweden (irfan.ahmad@umu.se; bernt.eric.uhlin@umu.se)
Reviewer: Philip Rather
Department of Microbiology and Immunology, EmoryUniversity, Atlanta, GA, USA (prather@emory.edu)
Overview of colony switching in Acinetobacter baumannii
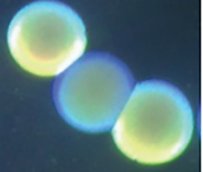
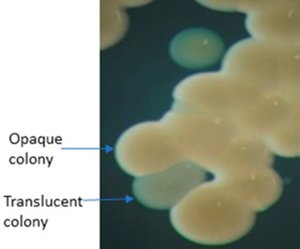
Acinetobacter baumannii isolates are displaying several features of heterogenicity. For example, there are motile/non-motile isolates, morphological variants such as bacilli/coccobacilli or filamentous/non-filamentous cells, and they can be encapsulated/non-capsulated. Recently, another interesting addition to the heterogenic features has been described as the appearance of opaque/translucent variants of colony morphotype on agar plates (Figures 1 & 2). It has been shown for several clinical isolates that A. baumannii has a remarkable ability to reversible switching between such colony morphotypes on agar plates that appear either opaque or translucent when viewed by a stereomicroscope (Tipton et al., 2015; Ahmad et al., 2019). The magnitude of opacity and frequency of switching vary from isolate to isolate (Ahmad et al., 2019). The opaque/translucent colony morphotype switching is associated with several clinically and environmentally relevant behavioural changes in A. baumannii. The opaque variant was shown to be virulent whereas the translucent variant appeared to be avirulent in experimental mouse models (Chin et al., 2018). Moreover, opaque variants formed a thicker capsule, efficiently escaped from immune cells and exhibited better capacity to tolerate disinfectants (Tipton et al., 2015; Chin et al., 2018). On the other hand, in Caenorhabditis elegans, used as a more environmentally relevant model of a predatory host organism, the translucent variant was shown to be causing reduced fertility of the worms and was thereby considered more virulent than the opaque counterpart (Ahmad et al., 2019).
Figure 1: colonies are viewed under normal room lighting or via oblique lighting from below the colonies. The translucent colony is flanked on either side by two opaque colonies (Tipton et al., 2015)
Figure 2: Several opaque and at least two translucent colonies of A. baumannii AB5075 visualized under stereomicroscope (Ahsan et al., 2022)
Regulation of colony switching
As far as the regulation of colony switching is concerned, much remain to be elucidated with respect to molecular mechanism(s). There is evidence that the phenomenon can be regulated in a cell density dependent manner that includes a secreted quorum sensing like molecule (Tipton et al., 2015). The TetR-type transcriptional regulators ABUW_1645 (Chin et al., 2018), ArpR (Tipton et al., 2017), and the LysR-type transcriptional regulator ABUW_1132 (Tierney et al., 2021) have been suggested as regulators of colony opacity in A. baumannii AB5075.
Early observations of switching between colonial forms of A. baumannii
(Ferguson & Roberts, 1950)
Future perspectives
Since the bacterium is very notorious for acute hospital infections and for its efficient capability to acquire antibiotic resistance and to survive over the treatment of disinfections there is much interest to find mechanisms that might be targeted as its “Achilles heel”. The switching capability can perhaps be considered as a strategic target in the quest to limit A. baumannii from its seemingly efficient ability to adopt to different environmental conditions. Hence, further insight into the regulatory mechanism(s) and in-depth knowledge about its in vivo implications are desirable to better understand the colony switching phenomenon.
References
Tipton KA, Dimitrova D, Rather PN. Phase-variable control of multiple phenotypes in Acinetobacter baumannii strain AB5075. J Bacteriol. 2015 Aug 1;197(15):2593-9.
doi: 10.1128/JB.00188-15
PMID: 26013481
Ahmad I, Karah N, Nadeem A, Wai SN, Uhlin BE. Analysis of colony phase variation switch in Acinetobacter baumannii clinical isolates. PLoS One. 2019 Jan 4;14(1):e0210082.
doi: 10.1371/journal.pone.0210082
PMID: 30608966
Chin CY, Tipton KA, Farokhyfar M, Burd EM, Weiss DS, Rather PN. A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii. Nat Microbiol. 2018 May;3(5):563-569.
doi: 10.1038/s41564-018-0151-5
PMID: 29693659
Ahsan U, Mushtaq F, Saleem S, Malik A, Sarfaraz H, Shahzad M, Uhlin BE, Ahmad I. Emergence of high colistin resistance in carbapenem resistant Acinetobacter baumannii in Pakistan and its potential management through immunomodulatory effect of an extract from Saussurea lappa. Front Pharmacol. 2022 Sep 16;13:986802.
doi: 10.3389/fphar.2022.986802
PMID: 36188613
Tipton KA, Farokhyfar M, Rather PN. Multiple roles for a novel RND-type efflux system in Acinetobacter baumannii AB5075. Microbiologyopen. 2017 Apr;6(2):e00418.
doi: 10.1002/mbo3.418
PMID: 27762102
Tierney ARP, Chin CY, Weiss DS, Rather PN. A LysR-type transcriptional regulator controls multiple phenotypes in Acinetobacter baumannii. Front Cell Infect Microbiol. 2021 Nov 4;11:778331.
doi: 10.3389/fcimb.2021.778331
PMID: 34805000
Ferguson WW, Roberts LF. A bacteriological and serological study of organism B5W (Bacterium antiratum). J Bacteriol. 1950 Feb;59(2):171-83.
doi: 10.1128/jb.59.2.171-183.1950
PMID: 15421945