Virulence
Author: Cecilia Ambrosi
Department of Human Sciences and Promotion of the Quality of Life, San Raffaele Roma Open University, Rome, Italy
(cecilia.ambrosi@uniroma5.it)
Reviewer: Chaoying Ma and Siobhán McClean
School of Biomolecular and Biomedical Sciences, University College Dublin, Belfield, Dublin, Ireland (chaoying.ma@ucdconnect.ie; siobhan.mcclean@ucd.ie)
Biofilm forming activity and environmental persistence
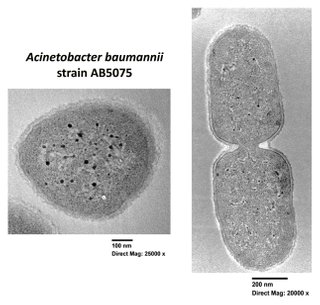
Clinical isolates of Acinetobacter baumannii can form biofilms on both biotic and abiotic surfaces; hospital settings and medical devices are the ideal environments for A. baumannii biofilms, thereby representing the main source of patient infections. Moreover, once it acquires the potential to form biofilms, A. baumannii has a remarkable ability to persist, resisting tenaciously even to long-term desiccation. Although environmental isolates collected from hospital surfaces are stronger biofilm producers compared to clinical strains associated with patients, the limited number of biofilm-eradicating treatments intensifies the need for effective anti-biofilm approaches. The main virulence factors involved in biofilms are illustrated in the figure and reported in the following sections. Transmission electron microscopy (TEM) images of a single and dividing cells of A. baumannii strain AB5075 are shown.
Magnification of two
A. baumannii cells embedded in extracellular polymeric biofilm
Schematic representation of A. baumannii surface structures involved in biofilm formation and maintenance, including AdeABC, RND efflux pump; Ata, autotransporter; Bap, biofilm-associated protein; BfmRS, two-component system; BlaPER-1, extended-spectrum β-lactamase; CUP, chaperone-usher pili; EPS, extracellular polymeric substance; IM, inner membrane; LPS, lipopolysaccharide; OM, outer membrane; OMPs, outer membrane proteins; OMVs, outer membrane vesicles; PNAG, poly-β-(1-6)-N-acetylglucosamine; T4SS, type IV secretion system; T6SS, type VI secretion system; Tuf, translation elongation factor; Wza-Wzb-Wzc system (Pompilio et al., 2021).
Transmission electron microscopy micrographs of A. baumannii AB5075 (Figure credit: Cecilia Ambrosi).
Host-pathogen interaction
Although only considered clinically relevant since the 1970s, ventilator-associated pneumonia (VAP) caused by A. baumannii is the leading cause of mortality in critically ill patients. A baumannii possesses both innate and acquired virulence factors including those involved in antibiotic resistance, environmental persistence and host-pathogen interactions. Consequently, studies on host-pathogen interactions focused on the ability to adhere to and invade host respiratory cells. Several virulence factors have been characterized to date. The outer membrane protein A (OmpA) plays a key role in A. baumannii pathogenesis. Other virulence factors, such as the oligosaccharide, diverse capsular polysaccharides, proteases and lipases (enzymes with hydrolytic and lipolytic activities), twitching motility assisted by type IV pili, micronutrient acquisition systems, moonlighting proteins, and different types of protein secretion systems, are the most studied. In addition, as with other pathogens, A. baumannii exploits host receptors as anchors for adhesion, establishing physical contact, mediating internalization via zipper mechanisms and stimulating host responses. The extraordinary variability of A. baumannii genomes makes the characterization of these virulence factors challenging. However, elucidation of the molecular mechanisms involved in virulence and persistence will lead us to the identification of effective targets to disarm this opportunistic pathogen.
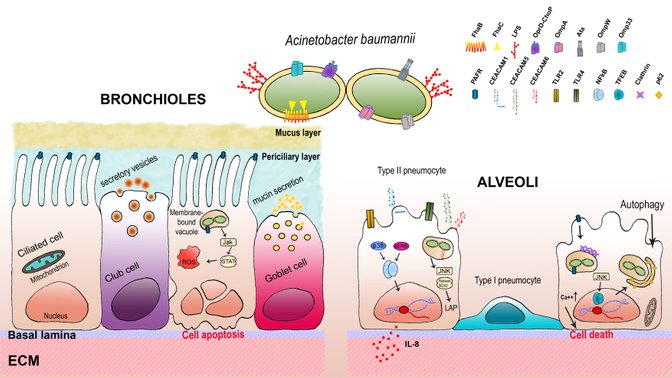
The virulence factors involved in A. baumannii interaction with the respiratory epithelium are illustrated in the figure. Virulence and persistence determinants defined so far in A. baumannii are reported in the following Table.
Schematic representation of the inter-relations between A. baumannii and the respiratory epithelium. A. baumannii has several virulence factors that enable the bacterium to adhere to and invade host cells. The main proteins involved in the interactions with host cells and extracellular host proteins are OmpA, Omp33, OmpW, Ata and FhaB. A. baumannii cells are internalized via a zipper mechanism where it can reside and survive within membrane-bound vacuoles. Bronchial epithelial cells respond to A. baumannii infections by eliciting the Jak-STAT pathway as well as the intracellular oxidative stress response that eventually leads to apoptosis. TLR2 and TLR4 on type II pneumocytes recognize lipoproteins and LOS and release cytokines (i.e., IL-8) via NF-κB and p38-Erk1/2-dependent pathway to chemoattract neutrophils at the site of infection. A. baumannii can engage CEACAM-1, -5, and -6 to gain access to type II pneumocytes. Internalization through CEACAM-1 triggers IL-8 production together with TLR2 and TLR4 via NF-κB and Erk1/2-dependent pathway; however, IL-8 secretion decreases significantly at 24 h post-infection, possibly due to a bacterial-induced inhibitory activity of CEACAM-1 ITIM on the TLR2 signalling cascade. Conversely, engagement of CEACAM-5 and -6 triggers LC3-associated phagocytosis (LAP) for clearance of A. baumannii via the JNK1/2-Rubicon-NOX2 pathway, which inhibits the canonical autophagic pathway. Furthermore, A. baumannii can interact with platelet-activating factor receptors (PAFRs) via ChoP-containing OprD, leading to a signalling cascade that includes G protein-coupled PLC, clathrin, β-arrestins and an increase of intracellular Ca2+, thereby leading to bacterial internalization. Invasion and persistence of A. baumannii are assisted by TFEB which induces the autophagosome-lysosome system that A. baumannii exploits to traffic intracellularly and persist within lung cells, possibly due to reduced lysosome acidification. It has been hypothesized that both intracellular persistence and apoptosis are A. baumannii strategies to allow bacterial dissemination to deeper tissues, thereby leading to invasive diseases. Individual components are not to scale (Sarshar et al. 2021).
Comprehensive list of all the known virulence factors of A. baumannii (last updated on 29.07.2022)
References
Ambrosi C, Scribano D, Sarshar M, Zagaglia C, Singer BB, Palamara AT. Acinetobacter baumannii targets human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) for invasion of pneumocytes. mSystems. 2020 Dec 22;5(6):e00604-20.
doi: 10.1128/mSystems.00604-20
PMID: 33361319
Bentancor LV, Camacho-Peiro A, Bozkurt-Guzel C, Pier GB, Maira-Litrán T. Identification of Ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J Bacteriol. 2012 Aug;194(15):3950-60.
doi: 10.1128/JB.06769-11
PMID: 22609912
Catel-Ferreira M, Marti S, Guillon L, Jara L, Coadou G, Molle V, Bouffartigues E, Bou G, Shalk I, Jouenne T, Vila-Farrés X, Dé E. The outer membrane porin OmpW of Acinetobacter baumannii is involved in iron uptake and colistin binding. FEBS Lett. 2016 Jan;590(2):224-31.
PMID: 26823169
Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, Kim SA, Lee SK, Lee JC. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005 Aug;7(8):1127-38.
doi: 10.1111/j.1462-5822.2005.00538.x
PMID: 16008580
Choi CH, Lee JS, Lee YC, Park TI, Lee JC. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 2008 Dec 10;8:216.
PMID: 19068136
Gautam D, Dolma KG, Khandelwal B, Mitsuwan W, Mahboob T, Pereira ML, Nawaz M, Wiart C, Ardebili A, Siyadatpanah A, Ehtesham H, Patra JK, Kwanhian W, Nissapatorn V. Acinetobacter baumannii: An overview of emerging multidrug-resistant pathogen. Med J Malaysia. 2022 May;77(3):357-370.
PMID: 35638493
Harding CM, Hennon SW, Feldman MF. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol. 2018 Feb;16(2):91-102.
PMID: 29249812
Kumar S, Anwer R, Azzi A. Virulence potential and treatment options of multidrug-resistant (MDR) Acinetobacter baumannii. Microorganisms. 2021 Oct 6;9(10):2104.
doi: 10.3390/microorganisms9102104
PMID: 34683425
Ma C, McClean S. Mapping global prevalence of Acinetobacter baumannii and recent vaccine development to tackle it. Vaccines (Basel). 2021 Jun 1;9(6):570.
PMID: 34205838
March C, Regueiro V, Llobet E, Moranta D, Morey P, Garmendia J, Bengoechea JA. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One. 2010 Apr 7;5(4):e10033.
doi: 10.1371/journal.pone.0010033
PMID: 20383325
Mea HJ, Yong PVC, Wong EH. An overview of Acinetobacter baumannii pathogenesis: Motility, adherence and biofilm formation. Microbiol Res. 2021 Jun;247:126722.
doi: 10.1016/j.micres.2021.126722
PMID: 33618061
Morris FC, Dexter C, Kostoulias X, Uddin MI, Peleg AY. The mechanisms of disease caused by Acinetobacter baumannii. Front Microbiol. 2019 Jul 17;10:1601.
PMID: 31379771
Parra-Millán R, Guerrero-Gómez D, Ayerbe-Algaba R, Pachón-Ibáñez ME, Miranda-Vizuete A, Pachón J, Smani Y. Intracellular trafficking and persistence of Acinetobacter baumannii requires transcription factor EB. mSphere. 2018 Mar 28;3(2):e00106-18.
PMID: 29600279
Pérez A, Merino M, Rumbo-Feal S, Álvarez-Fraga L, Vallejo JA, Beceiro A, Ohneck EJ, Mateos J, Fernández-Puente P, Actis LA, Poza M, Bou G. The FhaB/FhaC two-partner secretion system is involved in adhesion of Acinetobacter baumannii AbH12O-A2 strain. Virulence. 2017 Aug 18;8(6):959-974.
doi: 10.1080/21505594.2016.1262313
PMID: 27858524
Pompilio A, Scribano D, Sarshar M, Di Bonaventura G, Palamara AT, Ambrosi C. Gram-negative bacteria holding together in a biofilm: The Acinetobacter baumannii way. Microorganisms. 2021 Jun 22;9(7):1353.
doi: 10.3390/microorganisms9071353
PMID: 34206680
Smani Y, Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Ibáñez-Martínez J, Pachón J. Platelet-activating factor receptor initiates contact of Acinetobacter baumannii expressing phosphorylcholine with host cells. J Biol Chem. 2012 Aug 3;287(32):26901-10.
PMID: 22689572
Smani Y, Dominguez-Herrera J, Pachón J. Association of the outer membrane protein Omp33 with fitness and virulence of Acinetobacter baumannii. J Infect Dis. 2013 Nov 15;208(10):1561-70.
PMID: 23908480
Sarshar M, Behzadi P, Scribano D, Palamara AT, Ambrosi C. Acinetobacter baumannii: An ancient commensal with weapons of a pathogen. Pathogens. 2021 Mar 24;10(4):387.
doi: 10.3390/pathogens10040387
PMID: 33804894
Shan W, Kan J, Cai X, Yin M. Insights into mucoid Acinetobacter baumannii: A review of microbiological characteristics, virulence, and pathogenic mechanisms in a threatening nosocomial pathogen. Microbiol Res. 2022 Aug;261:127057.
doi: 10.1016/j.micres.2022.127057
PMID: 35569319