Capsular Polysaccharides
Author: Johanna J Kenyon
Centre for Immunology and Infection Control, School of Biomedical Sciences, Faculty of Health, Queensland University of Technology, Brisbane, Australia (johanna.kenyon@qut.edu.au)
Reviewer: Thomas A Russo
Veterans Administration Western New York Healthcare System, Department of Medicine, Jacobs School of Medicine and Biomedical Sciences, University Buffalo, Buffalo, NY, United States (trusso@buffalo.edu)
Importance of the polysaccharide capsule
The outer envelope of the A. baumannii cell is coated by a packed layer of polysaccharides, known as the capsule. At the immediate interface between the cell and the environment (Fig. 1), the capsule (otherwise known as capsular polysaccharide or CPS) plays an important role in many biological processes of A. baumannii and is a major determinant of virulence (Talyansky et al., 2021). Capsule fundamentally acts as a protective barrier providing resistance to complement- and phagocyte-mediated killing driving host innate immune evasion (Russo et al., 2010, Lees-Miller et al., 2013; Talyansky et al., 2021), but also to external stresses including desiccation, disinfectants, and certain antimicrobials (Tipton et al., 2018, Geisinger & Isberg, 2015). As one of the outer-most features of the cell, the capsule is also a primary receptor for many A. baumannii-specific bacteriophage (Gordillo Altamirano et al., 2021, Knirel et al., 2020, Oliveira et al., 2017) and a target for specific vaccines (Yang et al., 2017) and immunotherapies (Nielsen et al., 2021). These non-antibiotic therapeutic strategies rely on recognition and specificity for epitopes in the capsule structure, hence identifying and characterising the many diverse forms that strains may produce is important.
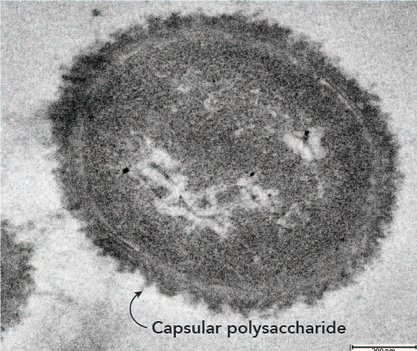
Transmission electron microscopy image of an A. baumannii AB5075 cell. Image courtesy of Johanna Kenyon (QUT, Australia). Note: Unlike many Gram-negative pathogens, A. baumannii does not produce an additional polysaccharide forming the O-antigen of the lipopolysaccharide (LPS) (Kenyon & Hall, 2013). Hence, the lipid A and core components are known as the lipooligosaccharide (LOS).
Structural diversity
Most A. baumannii isolates produce one of more than 100 distinct capsule types (Cahill et al., 2022, Wyres et al., 2020). These diverse structural formats exist as complex heteropolymers of repeating oligosaccharide glycan units, known as K-units, that can include between 2-8 sugars. The number, combination and order of sugars or non-carbohydrate constituents in the K-unit may differ between capsule types, as well as the glycosidic linkages between sugars or the K-units themselves (e.g. Kenyon et al., 2014, Kenyon et al., 2016, Arbatsky et al., 2022, Vinogradov et al., 2014). Interestingly, many capsular types have been found to include one of the nine known isomers of bacterial non-2-ulosonic acids. Three of these complex nine-carbon acidic sugars, acinetaminic acid (Kenyon et al., 2015), 8-epiacinetaminic acid (Kenyon et al., 2017) and 8-epipseudaminnic acid (Shashkov et al., 2022), are so far found exclusively in A. baumannii. While the role of different capsule types and relative pathogenicity has not been clearly established, some types have been associated with increased virulence in vivo (Talyansky et al., 2021) and/or poorer patient outcomes (Hsieh et al., 2021, Yang et al., 2022).
Genetics of capsule biosynthesis
Extensive diversity in A. baumannii capsular polysaccharides is primarily driven by the many possible combinations of genes that strains may carry at the chromosomal 'K locus' (Kenyon & Hall, 2013, Cahill et al., 2022, Wyres et al., 2020). Most genes required for capsule biosynthesis are clustered together at this location between the conserved fkpA and lldP genes (Kenyon & Hall, 2013). However, genes that influence the determination of the type of capsule produced have also been found in integrated genomic islands (Kenyon et al., 2016) or prophage regions (Arbatsky et al., 2022) elsewhere in the chromosome. Therefore, to simplify the ability to detect genetic differences at the K locus, and hence predict the type of capsule structure produced by an isolate, any new combination of genes found at this genomic location is assigned a unique KL identifier (e.g. KL1) and annotated using a standardised nomenclature system (Kenyon & Hall, 2013). More than 240 distinct KL have been described to date (Cahill et al., 2022), and are included in an international database of KL reference sequences (Cahill et al., 2022, Wyres et al., 2020). This database can be used to screen queried genome sequences for specific KL and other capsule biosynthesis genes in order to predict K type. It is available for public use and can be accessed via github, Kaptive-web and PathogenWatch.
References
Talyansky Y, Nielsen TB, Yan J, Carlino-Macdonald U, Di Venanzio G, Chakravorty S, Ulhaq A, Feldman MF, Russo TA, Vinogradov E, Luna B, Wright MS, Adams MD, Spellberg B. Capsule carbohydrate structure determines virulence in Acinetobacter baumannii. PLoS Pathog. 2021 Feb 2;17(2):e1009291.
doi: 10.1371/journal.ppat.1009291
PMID: 33529209
Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, Schultz LW, Umland TC, Campagnari AA. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 2010 Sep;78(9):3993-4000.
doi: 10.1128/IAI.00366-10
PMID: 20643860
Lees-Miller RG, Iwashkiw JA, Scott NE, Seper A, Vinogradov E, Schild S, Feldman MF. A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol Microbiol. 2013 Sep;89(5):816-30.
doi: 10.1111/mmi.12300
PMID: 23782391
Tipton KA, Chin CY, Farokhyfar M, Weiss DS, Rather PN. Role of capsule in resistance to disinfectants, host antimicrobials, and desiccation in Acinetobacter baumannii. Antimicrob Agents Chemother. 2018 Nov 26;62(12):e01188-18.
doi: 10.1128/AAC.01188-18
PMID: 30297362
Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015 Feb 13;11(2):e1004691.
doi: 10.1371/journal.ppat.1004691
PMID: 25679516
Gordillo Altamirano F, Forsyth JH, Patwa R, Kostoulias X, Trim M, Subedi D, Archer SK, Morris FC, Oliveira C, Kielty L, Korneev D, O'Bryan MK, Lithgow TJ, Peleg AY, Barr JJ. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat Microbiol. 2021 Feb;6(2):157-161.
doi: 10.1038/s41564-020-00830-7
PMID: 33432151
Knirel YA, Shneider MM, Popova AV, Kasimova AA, Senchenkova SN, Shashkov AS, Chizhov AO. Mechanisms of Acinetobacter baumannii capsular polysaccharide cleavage by phage depolymerases. Biochemistry (Mosc). 2020 May;85(5):567-574.
doi: 10.1134/S0006297920050053
PMID: 32571186
Oliveira H, Costa AR, Konstantinides N, Ferreira A, Akturk E, Sillankorva S, Nemec A, Shneider M, Dötsch A, Azeredo J. Ability of phages to infect Acinetobacter calcoaceticus‐Acinetobacter baumannii complex species through acquisition of different pectate lyase depolymerase domains. Environ Microbiol. 2017 Dec;19(12):5060-5077.
PMID: 29076652
Erratum in: Environ Microbiol. 2021 Jun;23(6):3334
Yang FL, Lou TC, Kuo SC, Wu WL, Chern J, Lee YT, Chen ST, Zou W, Lin NT, Wu SH. A medically relevant capsular polysaccharide in Acinetobacter baumannii is a potential vaccine candidate. Vaccine. 2017 Mar 7;35(10):1440-1447.
doi: 10.1016/j.vaccine.2017.01.060
PMID: 28190743
Nielsen TB, Yan J, Slarve M, Lu P, Li R, Ruiz J, Lee B, Burk E, Talyansky Y, Oelschlaeger P, Hurth K, Win W, Luna BM, Bonomo RA, Spellberg B. Monoclonal antibody therapy against Acinetobacter baumannii. Infect Immun. 2021 Sep 16;89(10):e0016221.
doi: 10.1128/IAI.00162-21
PMID: 34310884
Kenyon JJ, Hall RM. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLoS One. 2013 Apr 16;8(4):e62160.
doi: 10.1371/journal.pone.0062160
PMID: 23614028
Cahill SM, Hall RM, Kenyon JJ. An update to the database for Acinetobacter baumannii capsular polysaccharide locus typing extends the extensive and diverse repertoire of genes found at and outside the K locus. Microb Genom. 2022.
PMID: 36214673
Wyres KL, Cahill SM, Holt KE, Hall RM, Kenyon JJ. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb Genom. 2020 Mar;6(3):e000339.
PMID: 32118530
Kenyon JJ, Marzaioli AM, Hall RM, De Castro C. Structure of the K2 capsule associated with the KL2 gene cluster of Acinetobacter baumannii. Glycobiology. 2014 Jun;24(6):554-63.
PMID: 24688093
Kenyon JJ, Shneider MM, Senchenkova SN, Shashkov AS, Siniagina MN, Malanin SY, Popova AV, Miroshnikov KA, Hall RM, Knirel YA. K19 capsular polysaccharide of Acinetobacter baumannii is produced via a Wzy polymerase encoded in a small genomic island rather than the KL19 capsule gene cluster. Microbiology (Reading). 2016 Aug;162(8):1479-1489.
doi: 10.1099/mic.0.000313
PMID: 27230482
Arbatsky NP, Kasimova AA, Shashkov AS, Shneider MM, Popova AV, Shagin DA, Shelenkov AA, Mikhailova YV, Yanushevich YG, Hall RM, Knirel YA, Kenyon JJ. Involvement of a phage-encoded Wzy protein in the polymerization of K127 units to form the capsular polysaccharide of Acinetobacter baumannii isolate 36-1454. Microbiol Spectr. 2022 Jun 29;10(3):e0150321. doi: 10.1128/spectrum.01503-21
PMID: 35475638
Vinogradov E, Maclean L, Xu HH, Chen W. The structure of the polysaccharide isolated from Acinetobacter baumannii strain LAC-4. Carbohydr Res. 2014 May 22;390:42-5.
doi: 10.1016/j.carres.2014.03.001
PMID: 24690675
Kenyon JJ, Marzaioli AM, De Castro C, Hall RM. 5,7-di-N-acetyl-acinetaminic acid: A novel non-2-ulosonic acid found in the capsule of an Acinetobacter baumannii isolate. Glycobiology. 2015 Jun;25(6):644-54.
PMID: 25595948
Kenyon JJ, Notaro A, Hsu LY, De Castro C, Hall RM. 5,7-Di-N-acetyl-8-epiacinetaminic acid: A new non-2-ulosonic acid found in the K73 capsule produced by an Acinetobacter baumannii isolate from Singapore. Sci Rep. 2017 Sep 12;7(1):11357.
doi: 10.1038/s41598-017-11166-4
PMID: 28900250
Shashkov AS, Arbatsky NP, Senchenkova SN, Perepelov AV, Chizhov AO, Dmitrenok AS, Shneider MM, Knirel YA. NoteIdentification of 5,7-diacetamido-3,5,7,9-tetradeoxy-d-glycero-l-manno-non-2-ulosonic acid (di-N-acetyl-8-epipseudaminic acid) in the capsular polysaccharide of Acinetobacter baumannii Res546. Carbohydr Res. 2022 Mar;513:108531.
doi: 10.1016/j.carres.2022.108531
PMID: 35245711
Hsieh YC, Wang SH, Chen YY, Lin TL, Shie SS, Huang CT, Lee CH, Chen YC, Quyen TLT, Pan YJ. Association of capsular types with carbapenem resistance, disease severity, and mortality in Acinetobacter baumannii. Emerg Microbes Infect. 2020 Dec;9(1):2094-2104.
doi: 10.1080/22221751.2020.1822757
PMID: 32912064
Yang JL, Yang CJ, Chuang YC, Sheng WH, Chen YC, Chang SC. Association of capsular polysaccharide locus 2 with prognosis of Acinetobacter baumannii bacteraemia. Emerg Microbes Infect. 2022 Dec;11(1):83-90.
doi: 10.1080/22221751.2021.2011624
PMID: 34825848